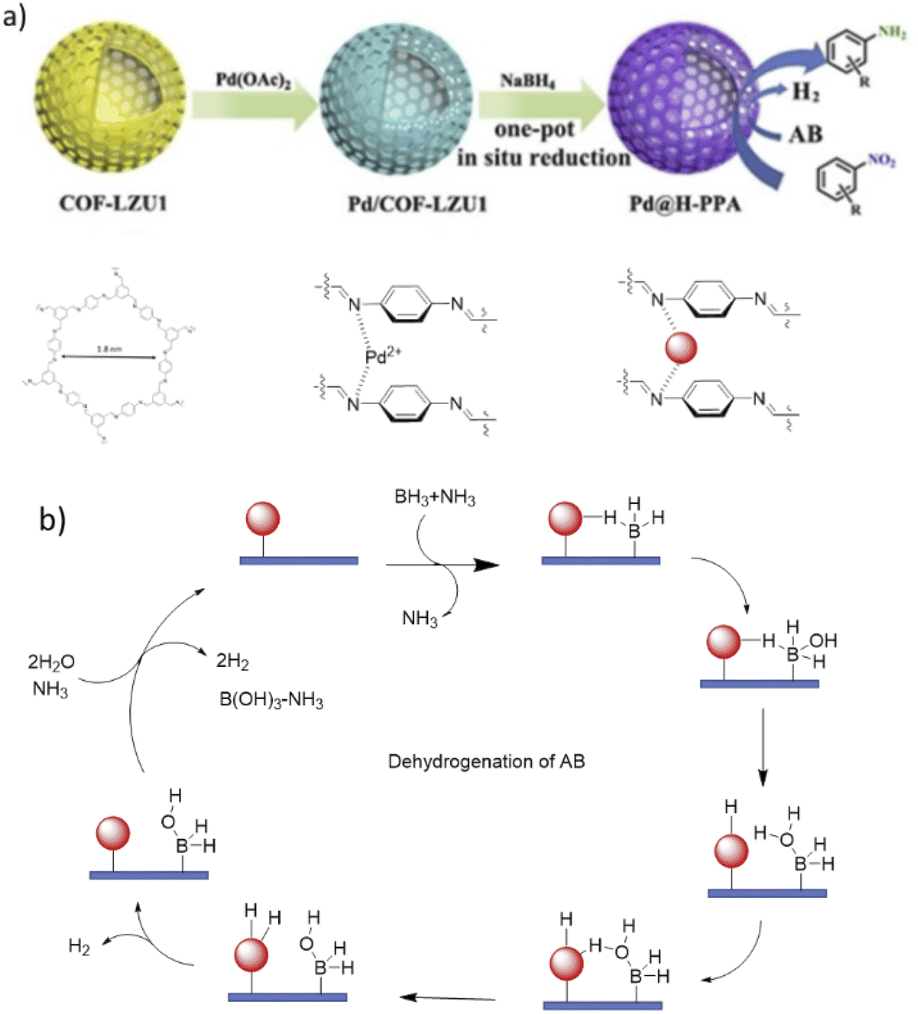
Covalent organic framework supported palladium catalysts - Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/D2TA05234B

Liquid‐ and Solid‐based Separations Employing Ionic Liquids for the Recovery of Platinum Group Metals Typically Encountered in Catalytic Converters: A Review - Lanaridi - 2022 - ChemSusChem - Wiley Online Library
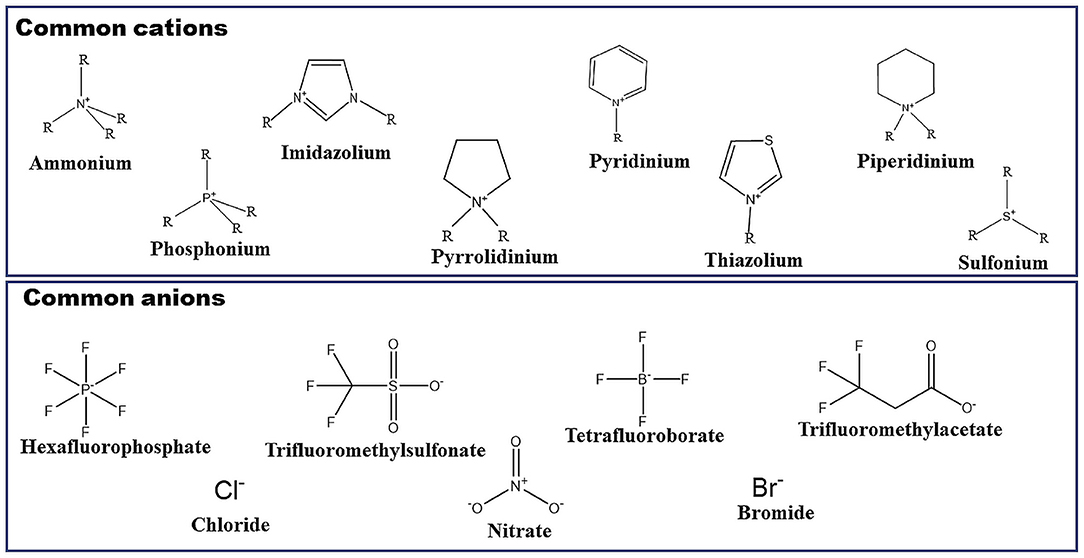
Frontiers | Scrutinizing Self-Assembly, Surface Activity and Aggregation Behavior of Mixtures of Imidazolium Based Ionic Liquids and Surfactants: A Comprehensive Review
![Optimization of Levulinic Acid Production from Depithed Sugarcane Bagasse in 1- Ethyl-3-methylimidazolium hydrogen sulfate [EMim][HSO4] | SpringerLink Optimization of Levulinic Acid Production from Depithed Sugarcane Bagasse in 1- Ethyl-3-methylimidazolium hydrogen sulfate [EMim][HSO4] | SpringerLink](https://media.springernature.com/lw685/springer-static/image/art%3A10.1007%2Fs12649-020-01221-z/MediaObjects/12649_2020_1221_Figa_HTML.png)
Optimization of Levulinic Acid Production from Depithed Sugarcane Bagasse in 1- Ethyl-3-methylimidazolium hydrogen sulfate [EMim][HSO4] | SpringerLink
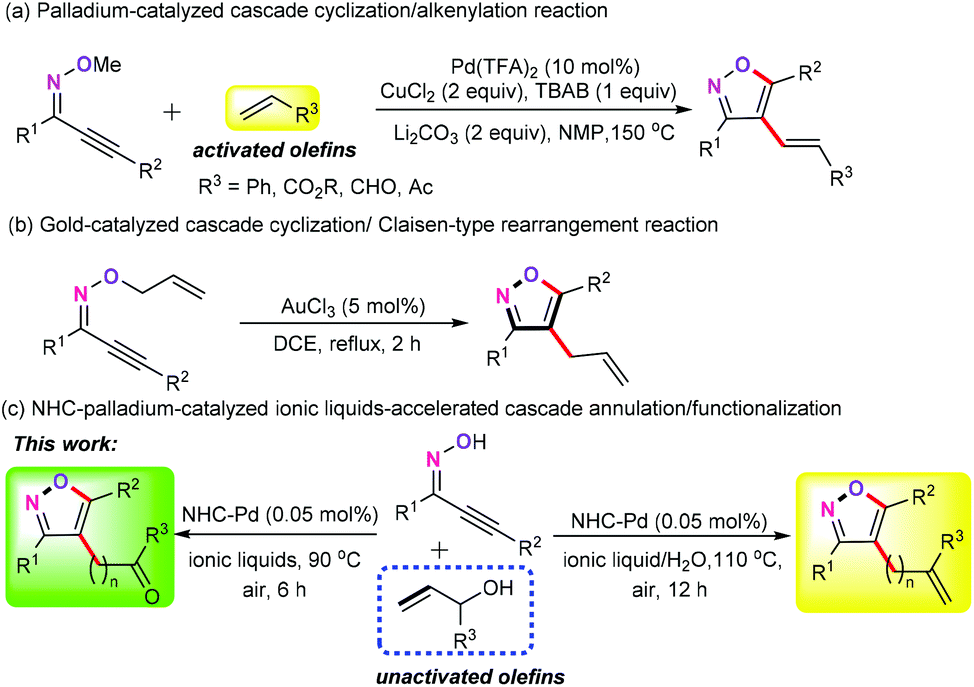
Palladium-catalyzed ionic liquid-accelerated oxidative annulation of acetylenic oximes with unactivated long-chain enols - Green Chemistry (RSC Publishing) DOI:10.1039/D0GC02037K

Palladium-Catalyzed Intramolecular 5-exo-dig Hydroarylations of N-Arylpropiolamides: Thermodynamics-Controlled Stereoselective Synthesis of 3-Methyleneoxindoles | The Journal of Organic Chemistry

Effect of chiral ionic liquids on palladium-catalyzed Heck arylation of 2,3-dihydrofuran - ScienceDirect

Mild Palladium-Catalyzed Selective Monoarylation of Nitriles | Journal of the American Chemical Society

Synthesis of Benzothiophene-3-carboxylic Esters by Palladium Iodide-Catalyzed Oxidative Cyclization–Deprotection–Alkoxycarbonylation Sequence under Aerobic Conditions - ScienceDirect

Electrochemical Palladium-Catalyzed Oxidative Sonogashira Carbonylation of Arylhydrazines and Alkynes to Ynones | Journal of the American Chemical Society

Mild Palladium-Catalyzed Selective Monoarylation of Nitriles | Journal of the American Chemical Society
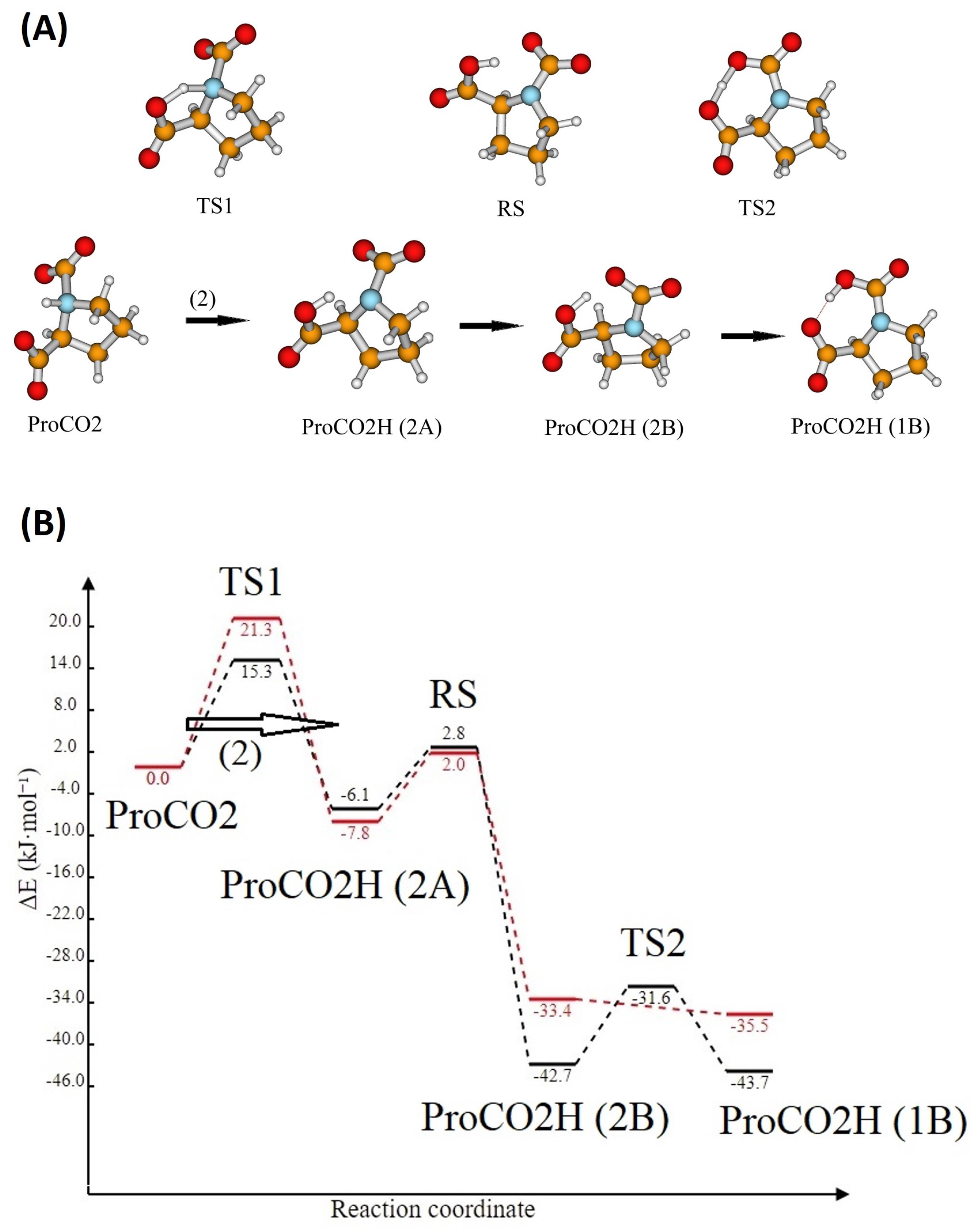
Entropy | Free Full-Text | Reaction Mechanism of CO2 with Choline-Amino Acid Ionic Liquids: A Computational Study

Mild Palladium-Catalyzed Selective Monoarylation of Nitriles | Journal of the American Chemical Society

1,3-Bis(carboxymethyl)imidazolium Chloride as a Metal-Free and Recyclable Catalyst for the Synthesis of N-Allylanilines by Allylic Substitution of Alcohols | ACS Sustainable Chemistry & Engineering
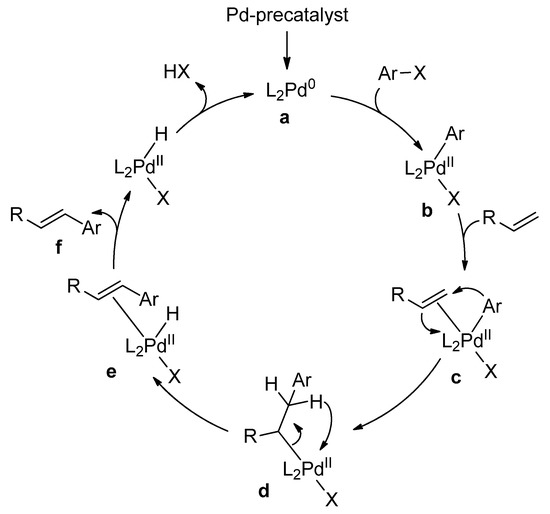
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond







![Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4] Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4]](https://www.degruyter.com/document/doi/10.1515/ncrs-2015-0117/asset/graphic/j_ncrs-2015-0117_fx_1.jpg)