Recent advances in the use of tri(2-furyl)germane, triphenylgermane and their derivatives in organic synthesis - ScienceDirect

Palladium labile precursors of 5FU (1) and gemcitabine (2). (b) FUdR... | Download Scientific Diagram

Recent advances in the use of tri(2-furyl)germane, triphenylgermane and their derivatives in organic synthesis - ScienceDirect
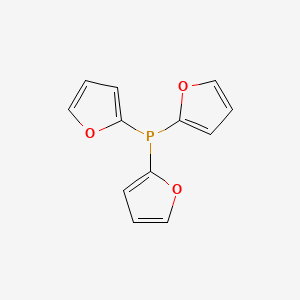
![5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation 5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/sc/01/5518-52-5.png)
5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation

WO2013000874A1 - Method for the preparation of palladium(i) tri-tert-butylphosphine bromide dimer and process for its use in isomerization reactions - Google Patents
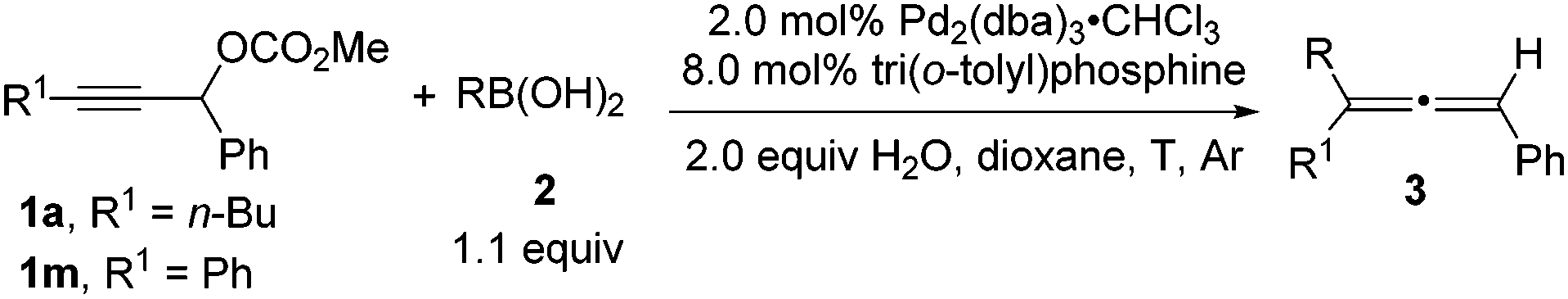
Tri(o-tolyl)phosphine for highly efficient Suzuki coupling of propargylic carbonates with boronic acids - Chemical Communications (RSC Publishing)

Recent advances in the use of tri(2-furyl)germane, triphenylgermane and their derivatives in organic synthesis - ScienceDirect

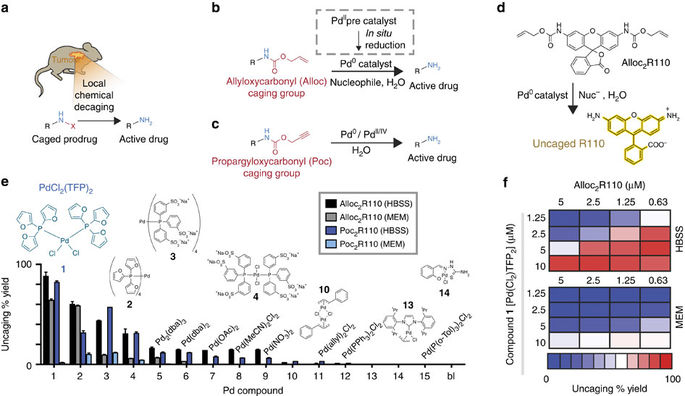
Palladium chemodosimeters based on change in optical properties. (a)... | Download Scientific Diagram

Palladium chemodosimeters based on change in optical properties. (a)... | Download Scientific Diagram

Carbonylation of terminal alkynes catalysed by Pd complexes in combination with tri(2-furyl)phosphine and methanesulfonic acid - ScienceDirect

Palladium‐Catalyzed Three‐Component Reaction of 3‐(Tri‐n‐ butylstannyl)allyl Acetates, Aldehydes, and Triorganoboranes: An Alternative to the Carbonyl Allylation Using α,γ‐Substituted Allylic Tin Reagents - Horino - 2016 - Advanced Synthesis & ...
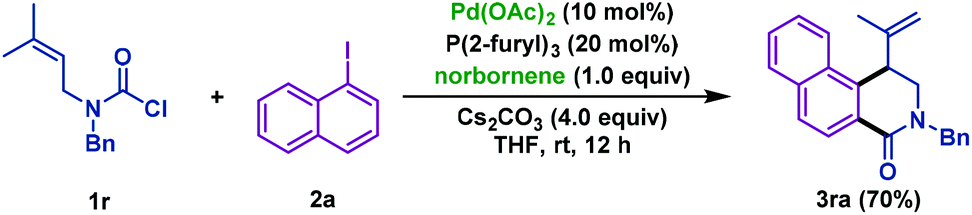
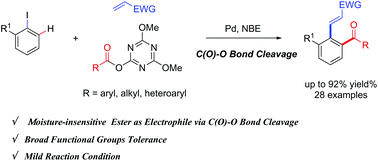
Pd/NBE-catalyzed sequential carbamoylation/olefination of aryl iodides - Organic Chemistry Frontiers (RSC Publishing)

China Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) CAS No.: 14221-01-3 Manufacturers - Free Sample - Alfa Chemical

Convenient and General Palladium‐Catalyzed Carbonylative Sonogashira Coupling of Aryl Amines - Wu - 2011 - Angewandte Chemie International Edition - Wiley Online Library

Palladium labile precursors of 5FU (1) and gemcitabine (2). (b) FUdR... | Download Scientific Diagram